Heteroepitaxy in Organic/TMD Hybrids and Challenge to Achieve it for TMD Monolayers: The Case of Pentacene on WS2 and WSe2 – Publication by A2 and B5
In their study published in ACS Applied Materials & Interfaces, Darius Günder, Marleen Axt and Gregor Witte reveal an epitaxial alignment of organic films on crystalline TMD substrates and demonstrate strategies for achieving this intrinsic van der Waals epitaxy, which is very sensitive to surface defects of the underlying 2D material, also for exfoliated monolayers.
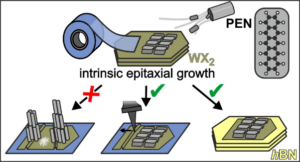
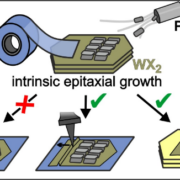
Comparison of different approaches to achieve the intrinsic epitaxy of PEN films on exfoliated and transferred TMD monolayers (Image: D. Günder, Reprinted with permission from ACS Appl. Mater. Interfaces 2024. Copyright 2024 American Chemical Society.)
The intriguing photophysical properties of monolayer stacks of different transition-metal dichalcogenides (TMDs) have recently prompted an extension of similar investigations on the interfacial excitonic coupling to hybrid systems of TMDs and organic films, as the latter combine large photoabsorption cross-section with the ability to tailor energy levels by targeted synthesis. In order to achieve such an excitonic coupling in momentum space a defined azimuthal alignment of the molecular adsorbate is crucial, which occurs on highly defined 2D material surfaces. However, this intrinsic van der Waals epitaxy of crystalline organic films cannot be automatically transferred to every 2D material surface because surface defects caused by exfoliation and transfer can result in significantly different film structures without any epitaxial order.
In this combined study of projects A2 and B5, Darius Günder et al. used X-ray diffraction, optical polarization, and atomic force microscopy to resolve the epitaxial alignment of crystalline pentacene (PEN) films grown at the basal plane of WS2 and WSe2 samples. While (022)-oriented PEN films with recumbent molecular orientation are formed on both studied TMDs, the azimuthal orientation of the long molecular axis is quite different. Moreover, it is shown that this intrinsic epitaxial growth of PEN films depends sensitively on the TMD surface quality. While it occurs on exfoliated TMD single crystals and multilayer flakes, it is hardly found on exfoliated and transferred monolayers, which often exhibit bubbles and wrinkles. This enhances the surface roughness and results in (001)-oriented PEN films with upright molecular orientation but without any azimuthal alignment. However, monolayer flakes can be smoothed by AFM operated in contact mode or by transferring TMD monolayers to ultrasmooth substrates such as hBN, which again yields epitaxial PEN films, but with significantly smaller domains than on TMD single crystals.
The presently demonstrated existence of epitaxial crystalline organic adlayers on TMDs paves the way for future investigations of interface or moiré excitons in such hybrid systems and also highlights the challenges in fabricating organic/TMD hybrid systems with well-defined interfaces.
Publication
D. Günder, M. Axt, G. Witte
Heteroepitaxy in Organic/TMD Hybrids and Challenge to Achieve it for TMD Monolayers: The Case of Pentacene on WS2 and WSe2
ACS Appl. Mater. Interfaces (2023) DOI:doi.org/10.1021/acsami.3c15829
Contact
Prof. Dr. Gregor Witte
Philipps-Universität Marburg
SFB 1083 project A2
Tel.: 06421 28-21384
EMAIL