Organic monolayers can reduce contact resistances in organic electronics – Publication by A2 (Witte)
In a detailed study, Felix Widdascheck, Alrun Hauke and Gregor Witte from SFB-project A2 show how phthalocyanine monolayers can be used to control the work function of noble metal electrodes, both in single crystalline model systems and for real life polycrystalline electrodes.
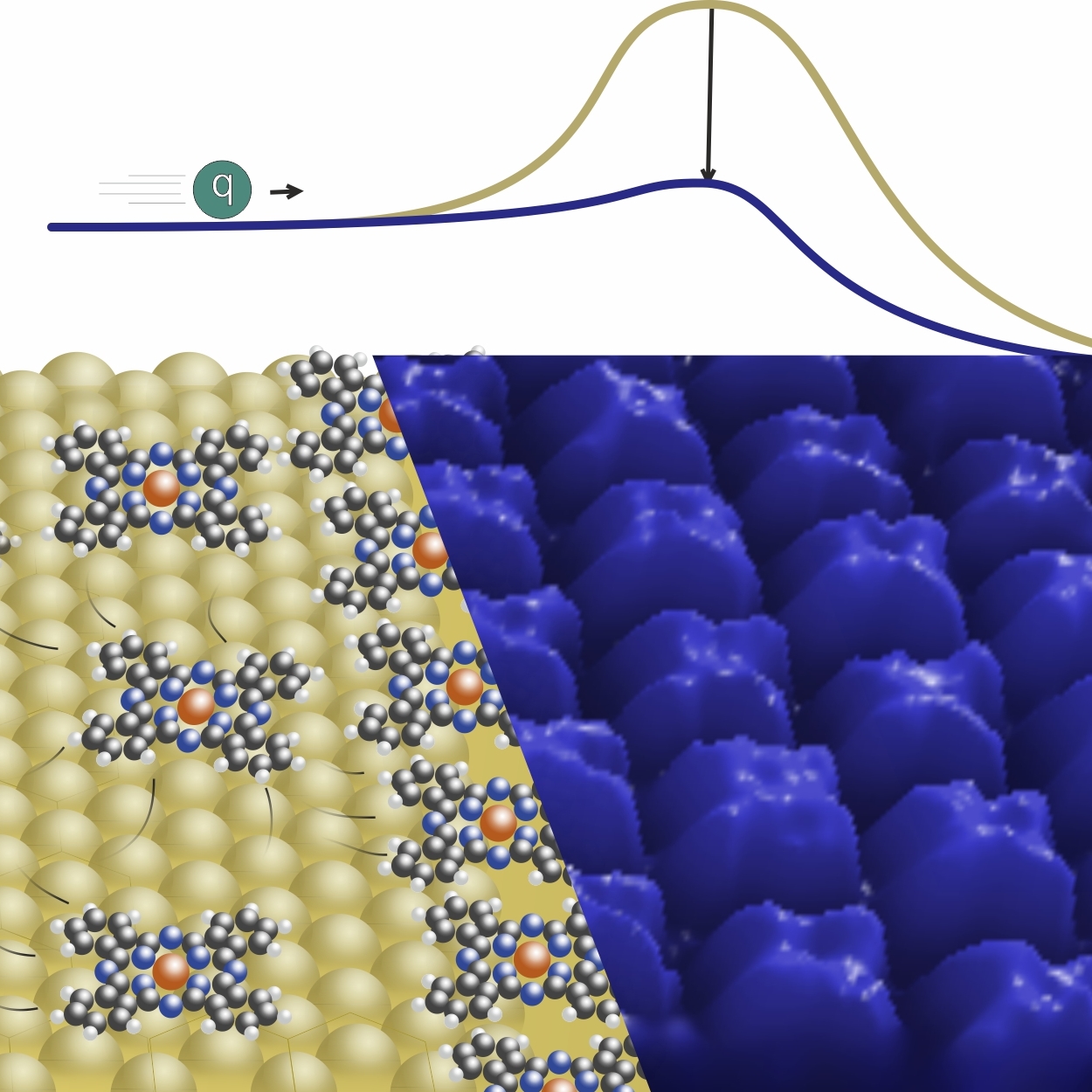
The work function of bare metal surfaces (yellow) can be modified by a thin layer of phthalocyanines (blue) to reduce injection barriers in organic electronic devices. (Image: F. Widdascheck).
Work function tailoring by means of organic monolayers is one of several promising approaches to reducing the contact resistance at the interface between metal electrodes and organic semiconductors in organic electronics devices.
In their study Felix Widdascheck and coauthors used several polar and non-polar phthalocyanines to modify the work functions of noble metal electrodes. As a starting point, they performed a detailed STM and Kelvin probe analysis of the coverage-dependent work function changes of Au and Ag single crystal surfaces. The authors find that the work function changes strongly depend on both coverage and the type of phthalocyanine used as the contact primer. Their phenomenological description of the observed trends provides important groundwork for more detailed theoretical modeling of the processes taking place at the complex internal interface between metal, monolayer and organic semiconductor.
In a further step towards actual device applications, the authors then transferred their findings and the developed preparation protocols to polycrystalline electrodes, demonstrating that the same work function changes can be observed also on “real-life” electrodes. With the end user in mind, the team also tested the air stability of their contact primers, proving that a sacrificial phthalocyanine multilayer serves well to protect the highly ordered mono- and bilayer contact primers during air transfer and can be removed by thermal desorption afterwards.
Publication
F. Widdascheck, A.A. Hauke and G. Witte,
A Solvent-Free Solution: Vacuum-Deposited Organic Monolayers Modify Work Functions of Noble Metal Electrodes
Adv. Funct. Mater. (2019) DOI: 10.1002/adfm.201808385
See also press release in German.
Contact
Prof. Dr. Gregor Witte
Philipps-Universität Marburg
SFB 1083 project A2
Tel.: 06421 28 21384
EMAIL