Molecular topology and the surface chemical bond: alternant versus nonalternant aromatic systems as functional structural elements
B.P. Klein, N.J. van der Heijden, S.R. Kachel, M. Franke, C.K. Krug, K.K. Greulich, L. Ruppenthal, P. Müller, P. Rosenow, S. Parhizkar, F.C. Bocquet, M. Schmid, W. Hieringer, R.J. Maurer, R. Tonner, C. Kumpf, I. Swart, J.M. Gottfried
Physical Review X 9 (2019) 011030
How an organic semiconductor bonds to a metal surface depends on the linking pattern of its carbon atoms. By comparing the aromatic, structurally isomeric, molecules naphthalene and azulene, it was shown that the unusual 5-7 ring system of azulene bonds much stronger to a metal surface than the 6-6 ring system of naphthalene. The knowledge gained from this model system is valuable in tuning the properties of metal-organic interfaces in organic electronic devices. It also sheds new light on how metals interact with graphene, in which azulene-like structural elements occur as defects.
In so-called organic electronic devices, such as modern displays with organic light-emitting diodes (OLEDs), organic materials connect to metal contacts. The resulting metal–organic interfaces determine important performance parameters such as rates of charge-carrier injection. Precise control over the interface properties, especially the wave-function overlap and the energy-level alignment, is therefore critical for developing improved devices. Here, several SFB 1083 projects together with international collaborators showed that the properties of metal-organic interfaces depend strongly on the topology, i.e., the linking pattern of the atoms in the p-electron system, of the organic molecule.

Naphthalene and azulene (a) are used as model systems for alternant and nonalternant structural elements. The 5-7 motif (blue) also occurs as a defect in graphene. The adsorption heights on Cu(111), as measured by the NI-XSW method (b), are compared in (c), revealing the much shorter adsorbate-substrate bond of azulene.
Organic semiconductors are typically based on adjoined hexagonal rings, like in graphene. This linking pattern is described as an alternant topology. Nonalternant topologies, which occur when the structure contains, for example, five- or seven-sided rings, have rarely been considered. To elucidate the influence of the topology on the interaction with a copper surface, the aromatic hydrocarbon naphthalene, which has an alternant topology, is compared with its nonalternant isomer, azulene.
This study reveals that azulene forms a much stronger and shorter bond to a copper surface than naphthalene. Spectroscopic analysis of the electronic structure shows that azulene forms a true chemical bond and receives negative charge from the surface, whereas naphthalene bonds only weakly and does not exchange charge. Theoretical analysis finds that the influence of the topology on the electronic structure, especially the lowest unoccupied molecular orbital (LUMO), is responsible for the different behavior.
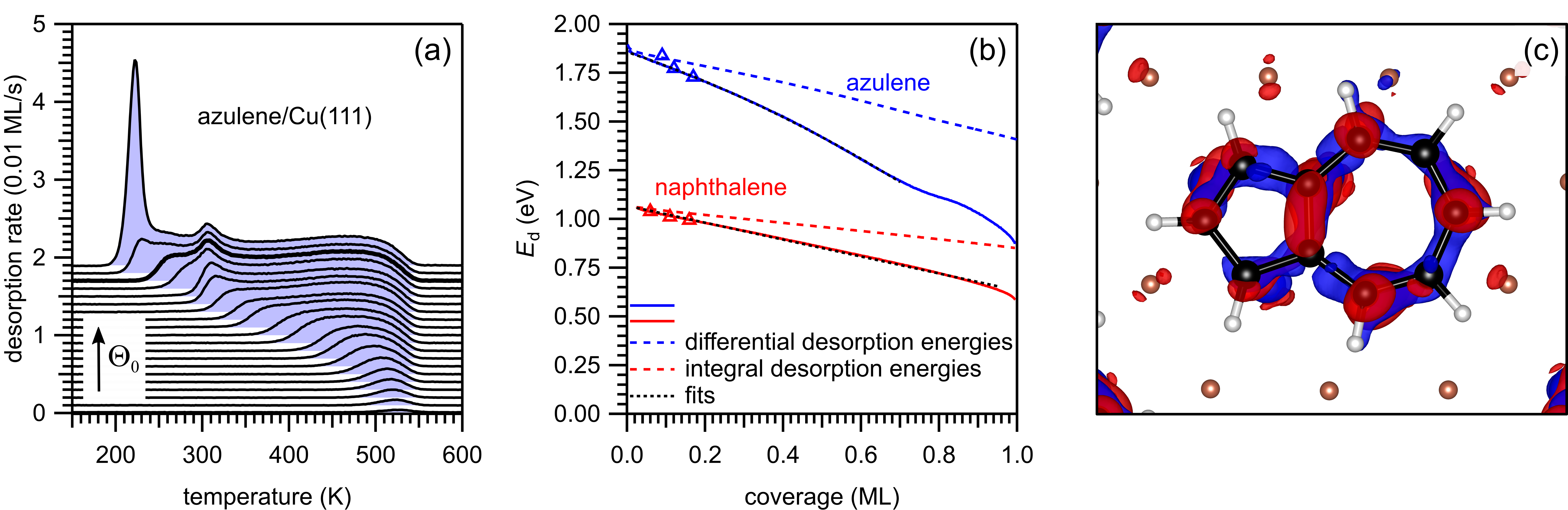
Analysis of the temperature-programmed desorption data in (a) confirms that azulene has a higher adsorbate-substrate interaction energy (b) over the whole monolayer coverage range. The bond of azulene to Cu(111) is associated with massive electron transfer from the surface to the LUMO of the molecule, as is illustrated in (c).
Based on these findings, it is proposed that nonalternant structural elements can be used to optimize performance-related properties of functional metal-organic interfaces. In addition, graphene defects with nonalternant topology, such as Stone-Wales defects, are expected to interact more strongly with metals than regular graphene, which is important for the electric contacting of carbon-based 2D materials in electronic devices.






