Ethers on Si(001): a prime example for the common ground between surface science and molecular organic chemistry
L. Pecher, S. Laref, M. Raupach, R. Tonner
Angewandte Chemie – International Edition 56 (2017) 15150
Material science and surface science have become research fields of ongoing importance pushing forward the development of new technologies and electronic devices. More recently, organic molecules have begun to be used, e.g., in the construction of organic light-emitting devices (OLEDs) or the organic functionalization of semiconductors. Chemical expertise is indispensable in describing bonding and reactivity phenomena in these fields. Especially on semiconductor surfaces, where electronic states are more localized compared to delocalized states on metals, the surface often behaves like a molecular reagent and solution chemistry concepts can be very helpful in describing the system.
The Si(001) surface is a widely used substrate due to its relevance for application and its high reactivity arising from both nucleophilic and electrophilic surface atoms being present. Ethers show an unexpectedly rich reactivity at the surface, a result that sparked extensive experimental investigation. Spectroscopy revealed a datively bonded (DB) intermediate and subsequent regioselective C-O bond breaking with a sizeable barrier. The surface-induced bond cleavage was proposed to stem from s*(C-O) occupation in the DB state facilitated by surface dimer flipping. These results render the system an ideal model for a theoretical approach.
The well-known surface reconstruction of Si(001) sees the formation of buckled dimers with an electronic structure well approximated by an empty p orbital at the Lewis acidic Sidown dimer atom and a non-bonding electron pair at the Lewis basic and nucleophilic Siup atom. Therefore, a mechanism analogous to the ether reaction known from molecular chemistry suggests itself: In the first step, a DB intermediate is formed between the oxygen and Sidown atoms while in the second step, any nearby nucleophilic Siup atom can attack a Cα atom to form a covalent Si-C bond.
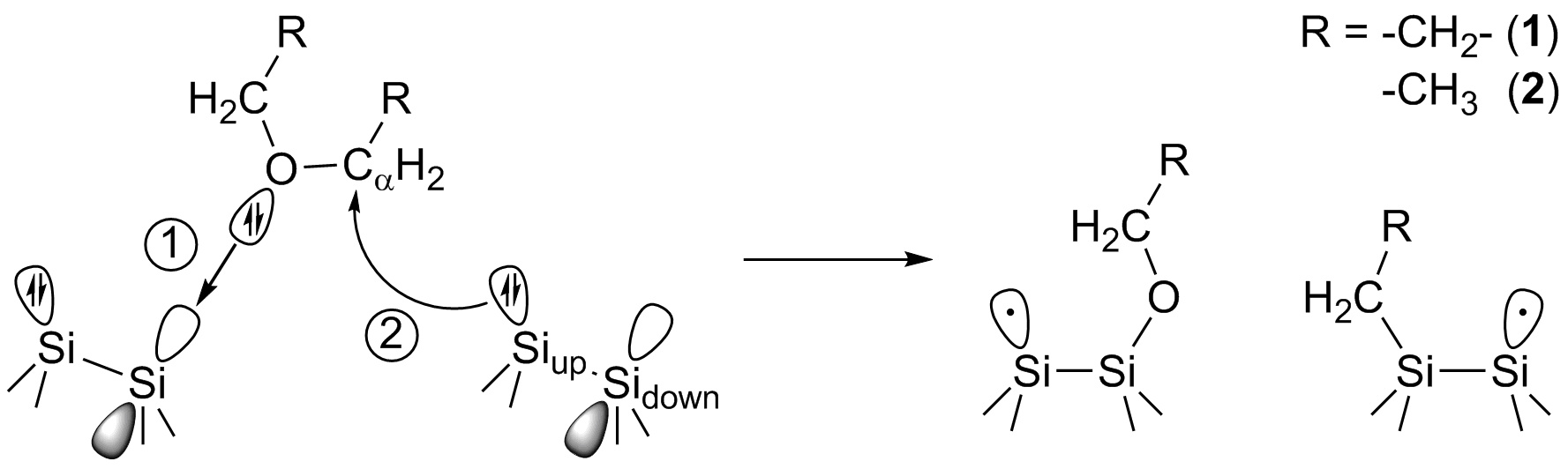
Two-step reaction of ether molecules with the Si(001) surface: 1) Formation of a dative bond between the ether oxygen atom and a Sidown surface atom through donation into the empty p orbital; 2) nucleophilic attack of a nearby Siup atom at Ca. The dots indicate unpaired electrons (dangling bonds).

a) Bonding analysis (pEDA) deformation density Dr1 at the DB structure of 1 (step 1) showing electron density transfer mainly from a p orbital shaped density at the molecule (red lobes) to the O-Si bonding region (blue lobes). b) LUMO of 1 in the TS(DB→C) geometry. c) pEDA deformation density Dr2 at the TS(DB→C) geometry (step 2) showing mainly how a s*(C-O) type density (blue lobes) is populated by electrons from the opposing surface dimer row (red lobes). Energies DEi in kJ mol–1, eigenvalues ni in qe.
This mechanism was proven by a quantitative analysis of the electronic structure in the surface-adsorbate system. By a novel approach of decomposing the bonding energy into well-defined physical terms (periodic energy decomposition analysis, pEDA), the main features of the reaction could be highlighted. It turned out that the datively bonded intermediate shows donation of the s- and p-type non-bonding electron pair into the formally empty surface state, thus explaining the high stability The regioselective ether cleavage is then understood as following a reaction pathway that closely resembles the nucleophilic reaction (SN2) known from textbook organic chemistry with bond-making and bond-breaking happening at the same time.
Thus, by quantitative electronic structure analysis the bonding, reactivity, and regioselectivity in this model system was proven to proceed very similarly to classical reactions of organic chemistry. This demonstrates that even under ultrahigh vacuum conditions on surfaces, which differ from the usual solvent-based conditions of chemistry, simple chemical concepts are applicable and allowing prediction. The reported nucleophilic substitution reaction can be expected to occur with any molecule having a Lewis basic group and a nearby carbon atom that can be attacked. This establishes nucleophilic substitution as a common class of surface reaction on semiconductor surfaces.






