Efficient syntheses of novel fluoro-substituted pentacenes and azapentacenes: molecular and solid-state properties
J. Schwaben, N. Münster, M. Klues, T. Breuer, P. Hofmann, K. Harms, G. Witte, U. Koert
Chemistry – A European Journal 21 (2015) 13758
Pentacene is one prototype of a π-aromatic building block in organic electronics. It is marked by its ability to form structurally defined films and inorganic/organic interfaces. In addition, pentacene’s stability can be increased by introducing nitrogen atoms into the π-aromatic system, while partial fluorination allows to introduce molecular dipole moments. With its highly-ordered and reproducible variability in the crystalline phase, pentacene and its derivates including azapentacene form important organic compounds.
In a combined experimental and computational approach, groups from chemistry and physics improved the synthesis of a range of novel partially fluorinated pentacene and azapentacene derivatives, at the same time providing full characterization of their properties ranging from crystal structure via optoelectronic properties to excitonic response.
One example for an efficient synthesis is the synthetic route to diaza-hexafluoropentacene 42 . An intermolecular amination of the diamine 8 with perfluoronaphthalene 39 gives the diamine 40. The subsequent intramolecular amination to produce the dihydrodiazapentacene 41 requires a careful adjustment of the base in order to prevent intermolecular side reactions. A final oxidation step then delivers the desired target compound 42.
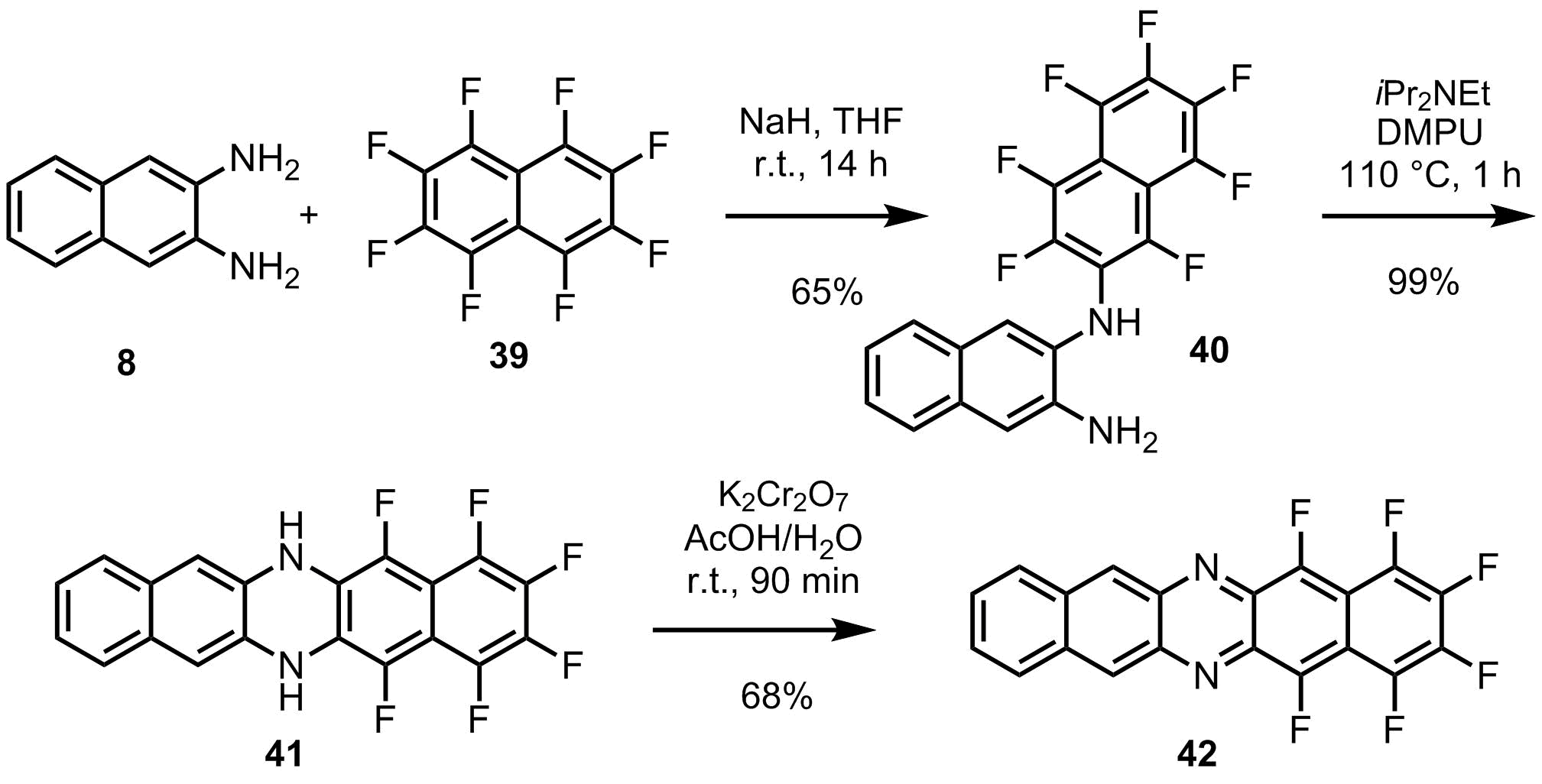
Chemical synthesis of the diaza-hexafluoropentacene 42.
In addition to the crystal structure analysis and the comparison of their UV / Vis absorption spectra in solution and in the solid state, the thin film growth was investigated. Using X-ray absorption (NEXFAS) measurements to determine the molecular orientation from the dichroism of the p* resonances it is shown for compounds 3, 30 and 42 that they adopt distinct recumbent and standing orientations on HOPG and SiO2 substrates. Notably, the structural analysis of the asymmetrically fluorinated compound 42 exhibits an alternating pacing motif that is stabilized by its molecular dipole moment.
These novel fluoro-substituted pentacenes and azapentacenes are interesting candidates for various organoelectronic devices like, for example, organic-light-emitting diodes (OLEDs), organic field-effect transistors (OFETs) and organic photovoltaics (OPVs).

Dichroic NEXAFS measurements of pentacene-type molecules 3, 42 and 30 (top left to right) on HOPG (middle, highly oriented pyrolitic graphite) and SiO2 (bottom) substrates. Inset pictures illustrate schematically the derived molecular orientation. (f) θ = 55° spectrum for compound 30 on SiO2. Inset indicates definition of different angles.






